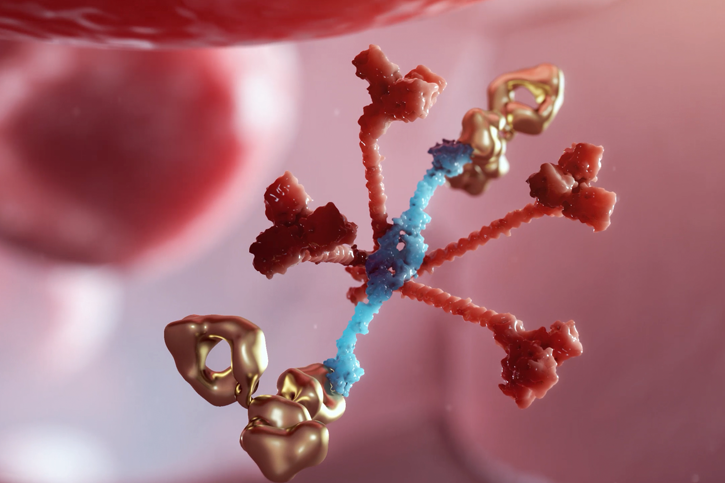
Narsoplimab (OMS721) is our lead antibody targeting MASP-2, the effector enzyme of the lectin pathway of complement.
Narsoplimab is a human monoclonal antibody targeting mannan-binding lectin-associated serine protease-2 (MASP-2), the effector enzyme of the lectin pathway of the complement system. The lectin pathway is one of the principal pathways of complement and is activated primarily by tissue damage and microbial infection. Importantly, inhibition of MASP-2 does not appear to interfere with the classical complement pathway, a critical component of the acquired immune response to infection. This novel, proprietary drug is designed to prevent complement-mediated inflammation and endothelial damage while leaving intact the respective functions of the other pathways of innate immunity.
1Khaled SK, Claes K, Goh YT, et al. Narsoplimab, a mannan-binding lectin-associated protease-2 inhibitor, for the treatment of adult hematopoietic stem-cell transplantation–associated thrombotic microangiopathy. J Clin Oncol. 2022; 40(22):2447-2457.
Narsoplimab
Narsoplimab (OMS721)
Hematopoietic Stem Cell Transplant-Associated Thrombotic Microangiopathy (TA-TMA)
2Whitaker S, Nangia N, Ng E, Sotolongo S, Pullman W. Systematic literature review of the natural history of hematopoietic stem cell transplant-associated thrombotic microangiopathy in adults. Presented at: 48th Annual Meeting of the European Society for Blood and Marrow Transplantation; March 19-23, 2022.Related Pages

